
Why do grilled dishes taste so good?

The science behind the process
Have you ever heard of the Maillard reaction? It was first described by the French chemist Louis-Camille Maillard in 1912, and it is also referred to as non-enzymatic browning. It affects the final taste of the products and the color of the food. Where can this phenomenon be observed? Welcome to the grill grate! Due to the Maillard reaction, steaks and sausages acquire a tasty, slightly caramel flavor. Scientists have noticed the relationship between the smell, taste, and color of the food during heat treatment.
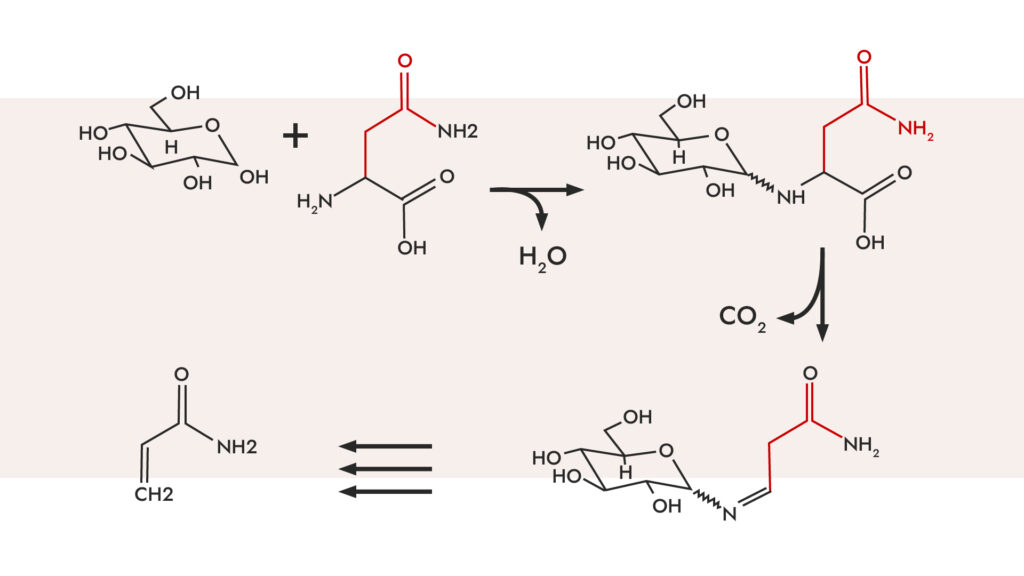
In simple terms, this reaction is a series of many simultaneous chemical reactions between simple sugars and amino acids. Hundreds of different flavor compounds are produced during the reaction which are then further broken down. It leads to the formation of brown food dyes. What steps does this reaction consist of?
1st step: it depends on the size of the reacting sugar molecule. The larger the sugar molecule, the slower the reaction with the amino acids will be. Compounds with a characteristic aroma, such as the smell of baking bread, may form at this stage.
2nd step: the breakdown of molecules and the formation of Amadori compounds and the formation of reactive fragrance compounds.
3rd step: dehydration and condensation of amino acids and the formation of smell.
The Maillard reaction is responsible for many colors and flavors in foods:
- caramel made from milk and sugar
- browning of toasted bread
- the color of beer, chocolate, coffee, and maple syrup
- the taste of roasted or grilled meat
- the color of condensed milk
The water and the temperature
The rate of the Maillard reaction is primarily influenced by two factors: the water content of the product and its processing temperature. Frying (and any other type of heat treatment above 100 degrees C – the boiling point of water) accelerates the Maillard reaction. At high temperatures, chemical reactions occur faster and water evaporates from the product faster. Interestingly, Maillard reactions take place already at room temperature, but they are so slow that we are unable to observe them – the product will spoil in time.
During the process, the products lose some carbohydrates and amino acids, which makes them less valuable for humans. Although the Maillard reaction has been studied for almost a century, its complex nature means that many partial reactions are still unknown.









Post a comment