
Caramelization – what is it?

We often say that something is caramelized, but do we know exactly what this means and how the process works? We will try to answer this question.
The sweet science
Caramel is a word that evokes good associations for most people around the world. Caramel apples, caramel candies, salted caramel – all these things increase the work of the salivary glands. However, caramelization is not a process that only applies to confectionery.
How does caramelization work? It is being studied and not fully understood. It’s a process in which sugar is subjected to high temperatures, which causes the water to evaporate completely. It gives the desired color and aroma to many products. Caramelization causes significant changes in food. Since no enzymes are involved in the caramelization process, it is a non-enzymatic browning reaction. An example of enzymatic browning will be the darkening of an apple or banana after cutting.
Research shows that we will find a total of over 600 substances in chocolate that have a beneficial effect on our health. These include magnesium, iron, potassium, zinc, copper, manganese, niacin, fiber, antioxidants, vitamins A, E, and omega-6 acids. There is a lot of calcium in milk and white chocolate. Dark chocolate is considered the healthiest.
Carbohydrates (also called sugars; compounds built of oxygen, hydrogen, and carbon) are found in almost all foods. Therefore, caramelization can be observed in many situations – when frying meat or vegetables (especially carrots or onions), and when baking bread (dark crust).
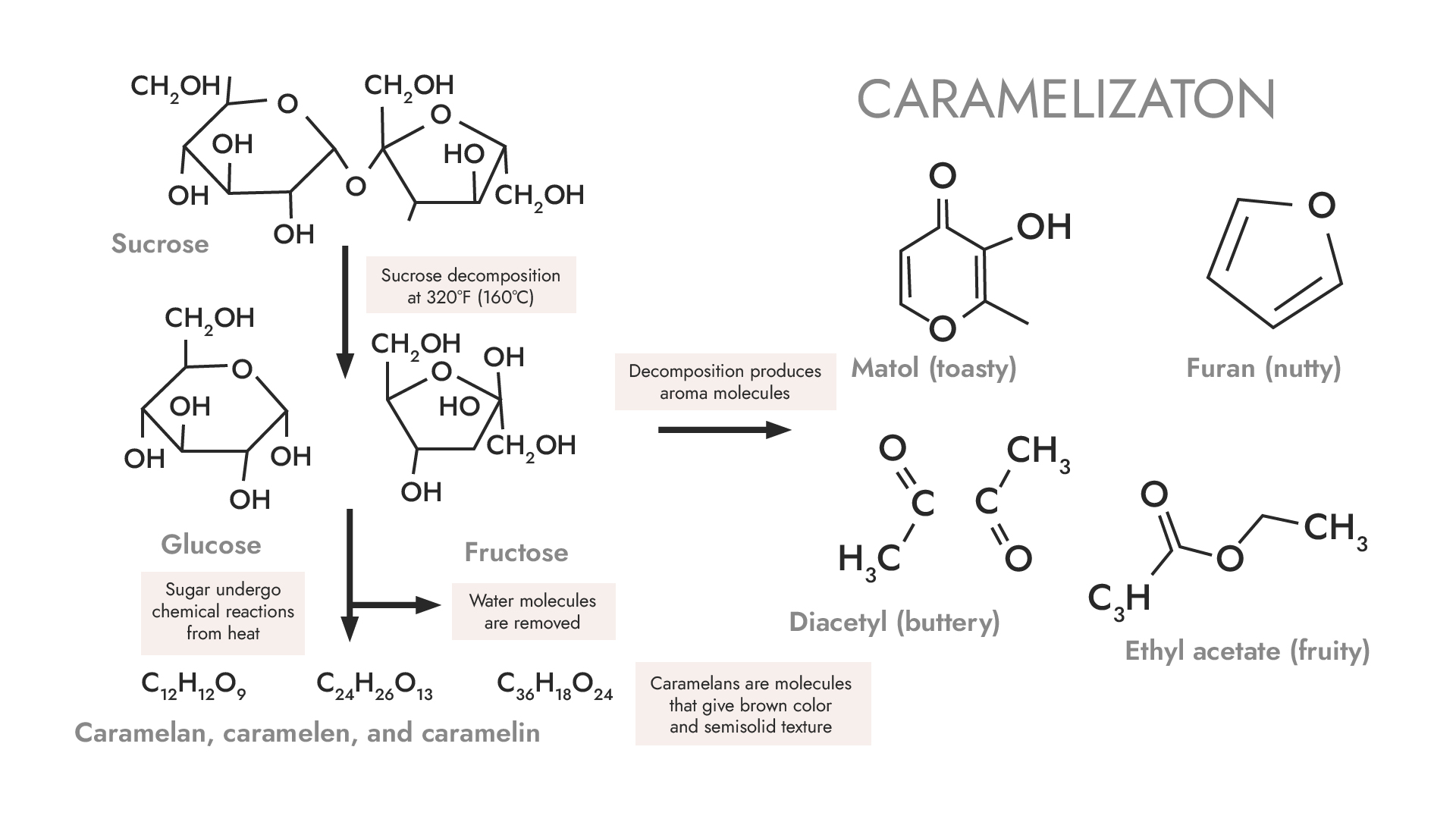
The incredible aroma
Aromatic compounds are also formed in the caramelization reaction and it is safe to say that probably most people love the smell of caramel. What is responsible for it? First of all, buttery diacetyl. Lactones provide creamy and fruity tones, while esters provide fruity sweetness. A characteristic compound of this process is the nut-bread 5-hydroxymethylfurfural (HMF). Maltol is responsible for the caramel notes. The sour smell is caused by the presence of acetic acid. These are just a few of the aromas that can be found in caramel. Its fragrance profile is extremely rich and varied. In addition to the aforementioned sweet, caramel, fruit, or nut flavors, we can sense flowers, coffee, or rum.
The caramelization starts at a much higher temperature compared to other browning reactions. The temperature of the caramelization start reaction depends on the type of sugar:
- Fructose – 110
- Galactose – 160 C.
- Glucose – 160 C.
- Sucrose – 160 C.
- Maltose – 180 C.
And where do these differences come from? Of course, from their chemical structure. The first three are monosaccharides, i.e. consisting of one sugar molecule.
The data above refer to pure substances. Several different carbohydrates are usually present in foods, as well as other ingredients that can affect caramelization temperature.
There are still some misteries
Despite the powerful legacy of science caramelization continues to be a poorly understood process.
Here is an overview of the steps of the general process:
- equilibration of anomeric and ring forms
- sucrose inversion to fructose and glucose
- condensation
- intramolecular bonding
- isomerization of aldoses to ketoses
- dehydration reactions
- fragmentation reactions
- unsaturated polymer formation
After all these scientific arguments, does anyone feel like having salted caramel ice cream? We do!









Post a comment